Synthesis technology and principle of sodium dodecylbenzene sulfonate
Sodium dodecylbenzene sulfonate is produced with n-dodecylbenzene as the main raw material, which is widely used as emulsifying and dispersing agent, antistatic agent and anti-caking agent for chemical products.
Name: Sodium dodecylbenzene sulfonate
Molecular formula:C12H25C6H4SO3Na
Molecular weight:348
Structure:
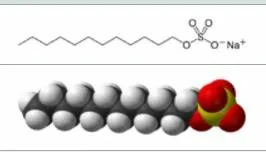
Its hydrophobic group is dodecyl phenyl group and hydrophilic group is sulfonic acid group. The branched chain of dodecyl is more detergency than the straight chain, and the branched chain has better solubility than the straight chain. Sodium dodecylbenzenesulfonate with branched chain is difficult to biodegrade, while straight chain sodium dodecylbenzenesulfonate is biodegradable.
Specification: Sodium salt solution with different concentrations (total solids ≤ 55%) is synthesized in dodecylbenzenesulfonic acid according to users’ needs. In addition to the active substance sodium dodecylbenzenesulfonic acid, there are inorganic salts (such as mannite, etc.), unsaponifiable substances (such as paraffinic hydrocarbons, advanced alkyl benzene, sulfone, etc.) and a large amount of water in the neutralization product. (In practice, users often prefer to buy dodecylbenzenesulfonic acid directly in order to adapt to the needs of different formulations, and then make further applications according to the characteristics of the product and the process.)
Synthesis route
Main raw materials: straight-run kerosene, hydrogen gas, benzene, anhydrous aluminum trichloride, sulfur trioxide, sodium hydroxide, etc.
The production line of sodium alkylbenzene sulfonate is shown below.
Production of alkyl benzene –sulfur trioxide sulfonation–sodium hydroxide neutralization–product
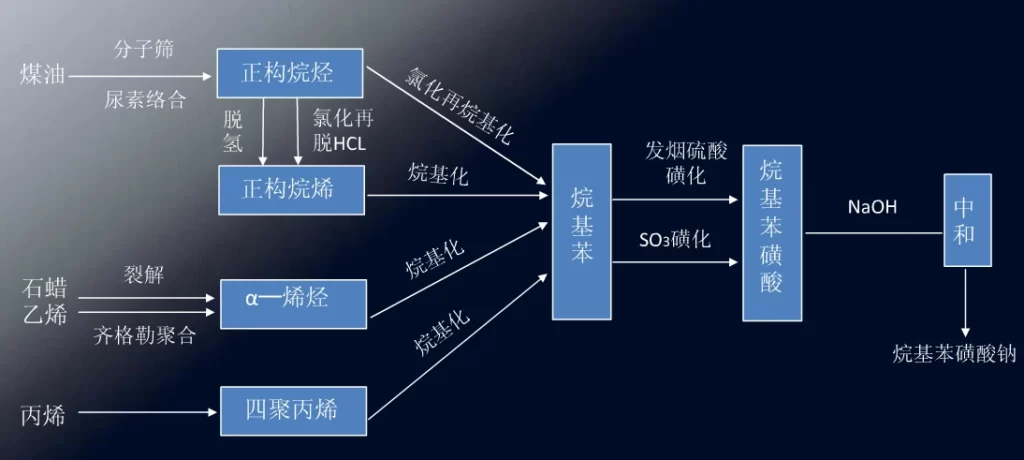
(1) kerosene feedstock route: this route is the most used, low cost of raw materials, mature process, product quality is also good.
(2) Paraffin cracking method.
(3) Ethylene Ziegler polymerization method: ɑ-olefin is first produced by route (2) and route (3), and alkylbenzene is obtained by reacting ɑ-olefin with benzene as an alkylation reagent. The alkyl benzene produced in this way is mostly 2-alkyl benzene, and the performance is not satisfactory when used as detergent.
(4) propylene zwitterionization method: propylene zwitterionization to get four polypropylene, and then alkylated with benzene, and then sulfonated, neutralized and highly branched sodium dodecylbenzene sulfonate (TPS). TPS is not easily biodegradable, causing environmental hazards, the 60s has been replaced by n-alkylbenzene, now only a small amount of production for pesticide emulsifiers.
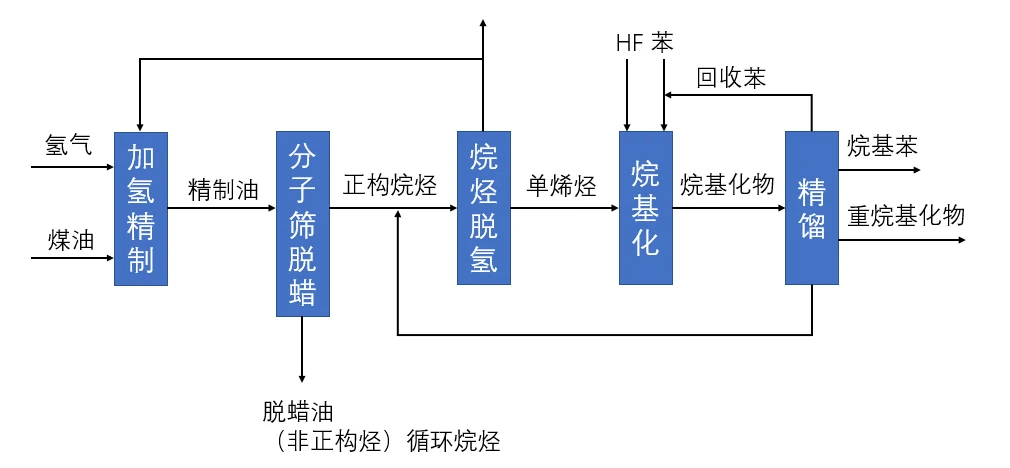
Preparation of alkylbenzene by dehydrogenation
Production principle: straight-run kerosene is hydrorefined and molecular sieve is dewaxed to produce n-alkanes, the n-alkanes in the travel chain are dehydrogenated to produce mono-olefins, and the mono-olefins and undehydrogenated n-alkanes react with benzene in the presence of catalyst to produce alkylbenzene
Production raw materials: straight-run kerosene, hydrogen, benzene, anhydrous aluminum trichloride
Advantages: more advanced process, better product quality, less amount of 2-substituted alkylbenzene
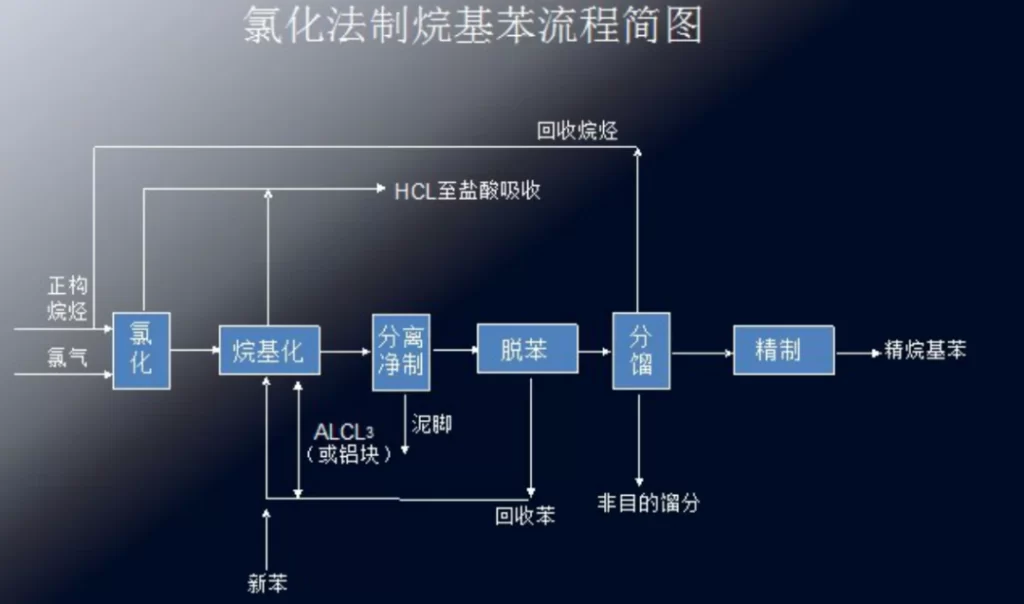
Preparation of alkylbenzene by chlorination
Raw materials, advantages and disadvantages and principles of alkylbenzene production
| Alkyl benzene production method | Raw materials | Advantages | Disadvantages | Principle |
|---|---|---|---|---|
| Chlorination | n-alkanes, chlorine, benzene, anhydrous aluminum trichloride | Low energy consumption | Difficulty in recycling hydrochloric acid by-product | Chlorination of n-alkanes with chlorine gas to produce chlorinated alkanes, which are alkylated with benzene in the presence of aluminum trichloride as a catalyst to produce alkylbenzene |
| Dehydrogenation method | Directly distilled kerosene, hydrogen, benzene, anhydrous aluminum trichloride | The process is more advanced, the product quality is better, the amount of 2-bit alkyl dumb is less, the production process of indene, naphthalene content is low, and the corrosion of equipment is small | High energy consumption | The long chain n-alkanes are dehydrogenated to produce mono-olefins, and the mono-olefins and undehydrogenated n-alkanes react with benzene in the presence of catalyst to produce alkylbenzene. |
Commonly used sulfonation method
| SulfonationMethod | Raw materials | Advantages | Disadvantages | Production Principle |
|---|---|---|---|---|
| Chlorosulfonic acid sulfonation method | Dodecylbenzene, chlorosulfonic acid, anhydrous aluminum trichloride | The reaction conditions are mild, almost quantitative reaction with organic materials under suitable conditions, the HC generated can be discharged, which is conducive to complete reaction, less side reactions, high product purity and yield, no by-production of waste H2SO4, no pollution of the environment. | Chlorosulfonic acid is expensive, the molecular weight is large, the amount of sulfonating agent for introducing one SO3 molecule is large, the resulting HC is highly corrosive, the operation is complicated, and inert organic solvent is required for sulfonation, which limits the scope of the reaction. | Chlorosulfonic acid (HSO3CI) can be regarded as a complex of SO3.HC, and its sulfonation ability is second only to that of sulfur trioxide, but the reaction is more moderate than the latter. Under anhydrous conditions, aromatic hydrocarbons are slowly added to an equimolar ratio or a slight excess of chlorosulfonic acid for reaction, and aromatic sulfonic acid is obtained. |
| Sulfamic acid sulfonation method | Dodecylbenzene, sulfamic acid, anhydrous aluminum trichloride | The resulting product is colorless, transparent, odorless, and can be used as a raw material for daily-use chemicals, and the reaction is mild and easy to control. | The price of sulfamic acid is more expensive and the production cost is high | C12H25+NH2SO3HC→12H25–SO3NH4 |
| Sulfur trioxide sulfonation method | Dodecylbenzene, sulfur trioxide | Not generatedH2O, No large amount of waste acid, less three wastes; strong sulfonation ability, fast reaction; saving dosage, close to the theoretical amount, low cost. Reasonable economy, high product quality, low impurities; fast reaction speed, sulfonation completed in seconds, high equipment productivity | SO3is very active, the reaction is intense, the thermal effect is large and difficult to control; the resulting product has high viscosity, heat dissipation is difficult, easy to occur multi-sulfonation, oxidation and other by-products | C12H25C6H6+ SO3→C12H25C6H6S03H+H2O |
Sodium hydroxide neutralization
C12H25C6H6+NaOH→C12H25C6H6SO3Na+H2O
Process of sodium dodecylbenzene sulfonate synthesis
Straight-run kerosene, hydrogen, benzene, anhydrous aluminum trichloride, sulfur trioxide, sodium hydroxide
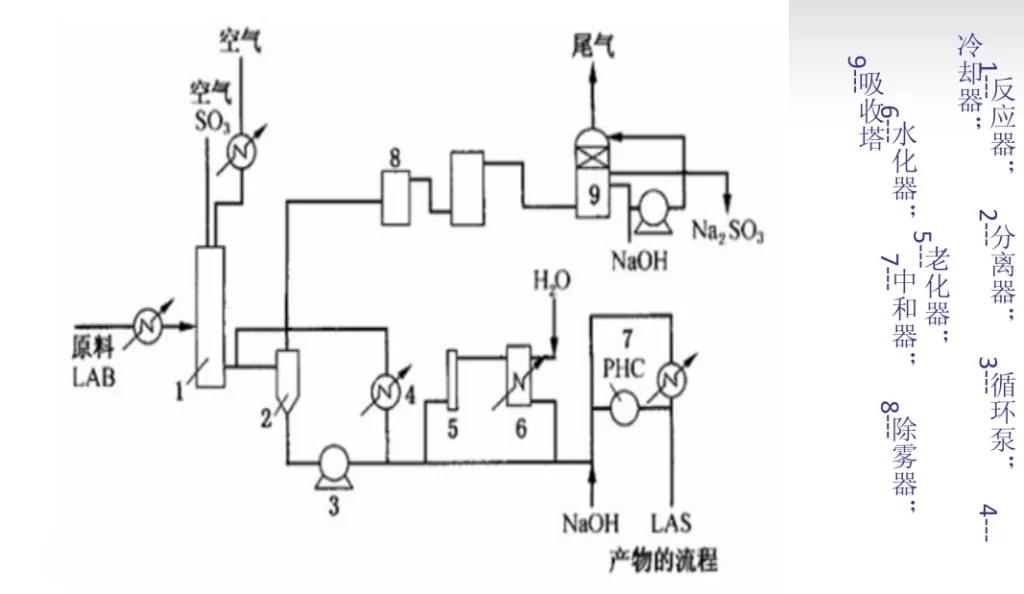
After the direct distillation kerosene is dehydrogenated, dodecaolefin and benzene are fed into the magician by the feed pump, and then the generated dodecylbenzene (LAB) is sent into the sulfonator to react with the sulfur trioxide (3%~5%) entering the sulfonator for instant sulfonation, and the product is treated by gas-liquid separator 2, circulation pump 3, and cooler 4, and then partly returned to the bottom of the reactor for rapid cooling of sulfonic acid, and partly the reaction product is sent into the aging apparatus 5 to adjust the Some of the reaction products are sent to the aging apparatus 5, and the reaction holding time is adjusted before entering the hydrator 6 to form acid, and finally the sodium alkyl benzene sulfonate (LAS) is made by the neutralizer 7. The exhaust gas is removed from the acid mist by mist eliminator 8, and the stock is absorbed by absorption tower 9 and then emptied.
Control of process conditions
The alkylation reaction uses aluminum trichloride as the catalyst, the reaction pressure is 0.6~0.8MPa, the temperature is 30~40℃, the molar ratio of benzene to olefin is about 10. sulfur trioxide is diluted to 3%~5% by dry air before entering the sulfonator, the purpose is to control the reaction speed and reduce the defects caused by the reaction speed. The molar ratio of sulfur trioxide to alkyl benzene is 1:1.03~1.05, and the reaction temperature is controlled at 25℃ not exceeding 30℃. Sodium hydroxide was prepared into a 10% solution and passed into the neutralizer to control the pH at 7~8. The neutralizer required constant stirring and the temperature was controlled at 40-50°C.
Exhaust gas treatment
The exhaust gas from the sulfonation reactor, besides air, also contains a small amount of acid mist and traces of SO3 and SO2 gas, which enters the electrostatic mist eliminator to remove the acid mist under the action of strong electric field. No waste water or solid waste is generated.
Process Principle
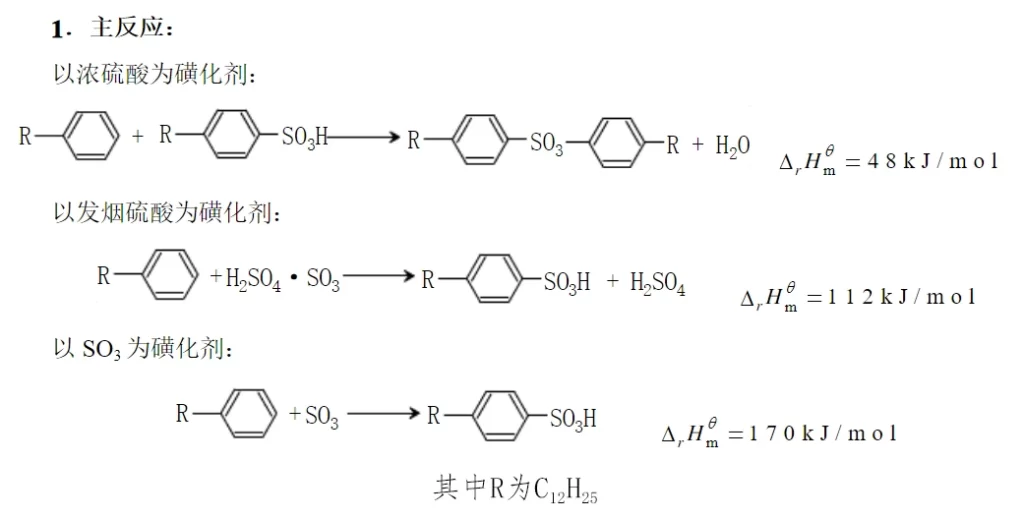
Sodium dodecylbenzene sulfonate is produced by the sulfonation reaction of straight-chain dodecylbenzene. The sulfonating agent can be concentrated sulfuric acid, fuming sulfuric acid and sulfur trioxide. The sulfonation reaction is an electrophilic substitution reaction. The sulfonating agent lacks electrons and is cationic, so it can easily attack the benzene molecule with affinity properties, and it is easy to have a substitution reaction on the benzene ring where the electron cloud density is large, accepting electrons, forming covalent bonds, and having a substitution reaction with the hydrogen on the benzene ring.
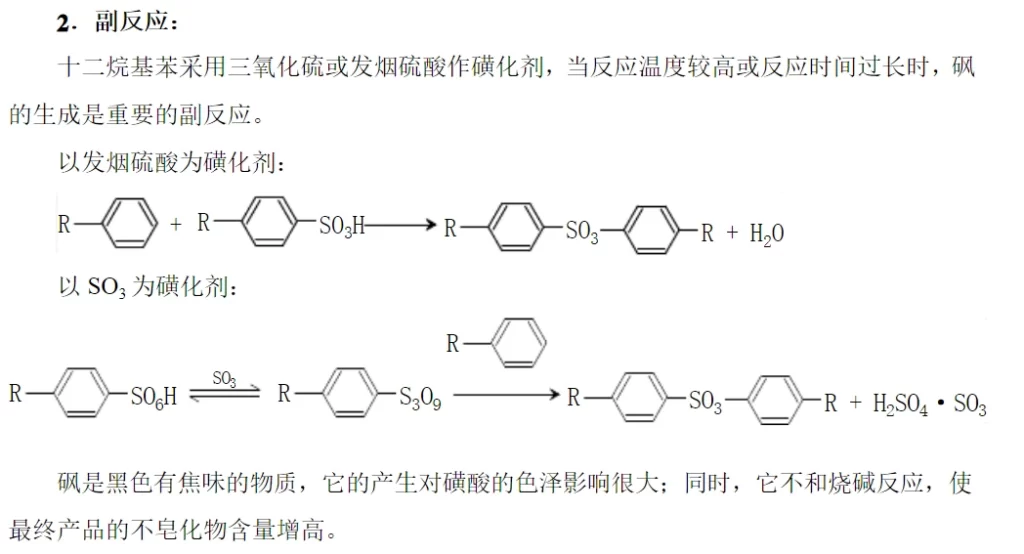
Due to the influence of the type of sulfonating agent, the nature of the object to be sulfonated and the reaction conditions, some sulfonating agents (e.g. fuming sulfuric acid) are themselves strong oxidizing agents, so there are a series of secondary side reactions (tandem reactions) and parallel side reactions occurring while the main reaction is going on, and the situation is very complicated. When straight-chain alkylbenzenes are sulfonated, the main side reaction is the formation of sulfone when the reaction temperature is too high or the reaction time is too long.
Application of products
Sodium dodecylbenzene sulfonate is a white or light yellow slightly viscous substance, commonly used in detergents and textile industry. It is an anionic surfactant. It is easily soluble in water and has good compatibility with anionic and nonionic compounds, with good emulsification, foaming, penetration, decontamination and dispersion properties, and is widely used in toothpaste, shampoo, shampoo, laundry detergent, liquid washing, cosmetics and plastic demoulding, lubrication and pharmaceutical, paper making, building materials, chemical and other industries.
[Application 1] Used as detergent and textile auxiliary, also used as toothpaste foaming agent, mine fire extinguishing agent, emulsion polymerization emulsifier, wool purifying agent, etc.
[Use 2] Used as anionic surface activator, emulsifier and foaming agent
[Use 3] GB 2760-96 stipulates that it is a processing aid for food industry. Foaming agent; emulsifier; anionic surfactant. Used in cakes, beverages, egg whites, fresh fruits, juice drinks, edible oils, etc.
[Use 4] Used as emulsifier for drugs, cosmetics and synthetic resin. Used as foaming agent for toothpaste and fire extinguisher. Used as a detergent for silk and wool fine fabrics. Flotation agent for metal beneficiation.
[Use 5] Used as washing and textile auxiliary, also used as toothpaste foaming agent, fire extinguishing foam, emulsifier for emulsion polymerization, emulsifying and dispersing agent for medicine, shampoo and other cosmetic products, wool purifying agent.
【Use 6】Biochemical analysis, electrophoresis, ion-pairing reagent.
*Disclaimer: The content contained in this article comes from the Internet, WeChat public numbers and other public channels, and we maintain a neutral attitude toward the views expressed in the article. This article is for reference and exchange only. The copyright of the reproduced manuscript belongs to the original author and the institution, and if there is any infringementPlease contact Jetson Chemical for deletion
