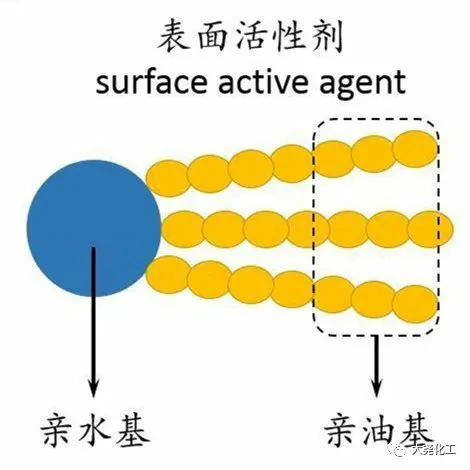
The antibacterial mechanism of baryonic surfactant is expected to be effective in killing bacteria. The term surfactant comes from the English word Surfactant, which is a contraction of the phrases Surface (surface), Active (active), and Agent (additive). Surfactant is a substance that is active on surfaces and interfaces and has a very high ability and efficiency in reducing surface (boundary) tension, forming molecularly ordered assemblies in solutions above a certain concentration and thus having a range of application functions. Surfactants possess good dispersibility, wettability, emulsification ability, and antistatic properties, and have become key materials for the development of many fields, including the fine chemical industry, and have significantly contributed to the improvement of processes, reduction of energy consumption, and improvement of production efficiency. With the development of society and the continuous progress of the world’s industrial level, the application of surfactants has gradually spread from daily-use chemicals to various fields of the national economy, such as antibacterial agents, food additives, new energy fields, pollutant management and biopharmaceuticals.
The special structure of the baryonic surfactant results in a high surface activity, which is mainly due to.
(1) The two hydrophobic tail chains of the baryonic surfactant molecule result in enhanced hydrophobicity and an increased tendency for the surfactant to leave the aqueous solution.
(2) The tendency of hydrophilic head groups to separate from each other, especially ionic head groups due to electrostatic repulsion, is substantially weakened by the influence of the linking group.
(3) The special structure of baryonic surfactants affects their aggregation behavior in aqueous solution, giving them more complex and variable aggregation morphology.
Baryonic surfactants have higher surface (boundary) activity, lower critical micelle concentration, better wettability, emulsification ability and antibacterial ability than conventional surfactants. Therefore, the development and utilization of baryonic surfactants are of great significance for the development and application of surfactants.
The “amphiphilic structure” of conventional surfactants gives them unique surface properties. When a conventional surfactant is added to water, the hydrophilic head group tends to dissolve inside the aqueous solution, while the hydrophobic group inhibits the dissolution of surfactant molecules in water. Under the combined effect of these two tendencies, surfactant molecules are enriched at the gas-liquid interface and undergo an ordered arrangement thereby reducing the surface tension of water. Unlike conventional surfactants, baryonic surfactants are “dimers” that link conventional surfactants together through spacer groups, which can reduce the surface tension of water and oil/water interfacial tension more effectively. In addition, baryonic surfactants have lower critical micelle concentrations and better water solubility, emulsification, foaming, wetting and antibacterial properties.
I. Cationic baryonic surfactants
Cationic baryonic surfactants can dissociate cations in aqueous solutions, mainly ammonium and quaternary ammonium salt baryonic surfactants. Cationic baryonic surfactants have good biodegradability, strong decontamination ability, stable chemical properties, low toxicity, simple structure, easy synthesis, easy separation and purification, and also have bactericidal properties, anticorrosion, antistatic properties and softness.
II. Anionic baryonic surfactants
Anionic baryonic surfactants can dissociate anions in aqueous solution, mainly sulfonates, sulfate salts, carboxylates and phosphate salts type baryonic surfactants. Anionic surfactants have better properties such as decontamination, foaming, dispersion, emulsification and wetting, and are widely used as detergents, foaming agents, wetting agents, emulsifiers and dispersants.
III. Non-ionic baryonic surfactants
Nonionic baryonic surfactants cannot be dissociated in aqueous solution and exist in molecular form. This type of baryonic surfactant has been less studied so far, and there are two types, one is sugar derivatives, and the other is alcohol ether and phenol ether. Non-ionic baryonic surfactants do not exist in the ionic state in solution, so they have high stability, are not easily affected by strong electrolytes, have good compounding with other types of surfactants and have good solubility. Therefore, nonionic surfactants have various properties such as good detergency, dispersibility, emulsification, foaming, wettability, antistatic property and sterilization, and can be widely used in various aspects such as pesticides and coatings.
*Disclaimer: The content contained in this article comes from the Internet, WeChat public numbers and other public channels, and we maintain a neutral attitude toward the views expressed in the article. This article is for reference and exchange only. The copyright of the reproduced manuscript belongs to the original author and the institution, and if there is any infringementPlease contact Jetson Chemical for deletion
